Background
Primary immune thrombocytopenia (ITP) is a rare acquired autoimmune disorder characterized by low platelet count, with an overall age/sex-standardized annual incidence of 6.1 per 100,000 persons in the US in 2016. Patients who fail to respond or maintain a response to initial therapy (e.g., corticosteroids, intravenous immunoglobulins), move to advanced therapies with robust evidence such as thrombopoietin receptor agonists (TPO-RAs [eltrombopag/ romiplostim/ avatrombopag]), rituximab, and fostamatinib, as per the international consensus guidelines. Among patients with primary ITP lasting ≥3 months who are first-time users of advanced therapies, there is limited real-world evidence on patient characteristics, treatment patterns, and clinical outcomes.
Objectives
The key objectives were to assess patient characteristics, treatment patterns, and clinical outcomes of first-time users of advanced therapy in patients with primary ITP lasting ≥3 months.
Methods
This retrospective, cohort study included patients identified from Optum de-identified Clinformatics ® Data Mart database, aged ≥18 years with primary ITP lasting 3 months or more, who initiated advanced therapy for the first time between October 1, 2015, and August 31, 2020. Patients were required to have ≥365 days of available data prior to index date, without evidence of conditions associated with secondary ITP. Patients were indexed on the date of initiation of advanced therapies.
Baseline characteristics, including demographics, comorbidities, use of medication, and bleeding events requiring medical attention (e.g., intracranial bleeding, gastrointestinal bleeding, hematuria) were assessed 365 days prior to index and reported using descriptive statistics. Treatment patterns and rates of bleeding events, thromboembolic events (TEs), bacterial infections, and hospitalizations were assessed during follow-up, which began one-day after index date and until discontinuation of the index therapy. Patients were censored on death, end of data accrual, disenrollment, or switch to another advanced therapy. Outcomes were reported as the number of events per 1000 person-years and time to first event.
Results
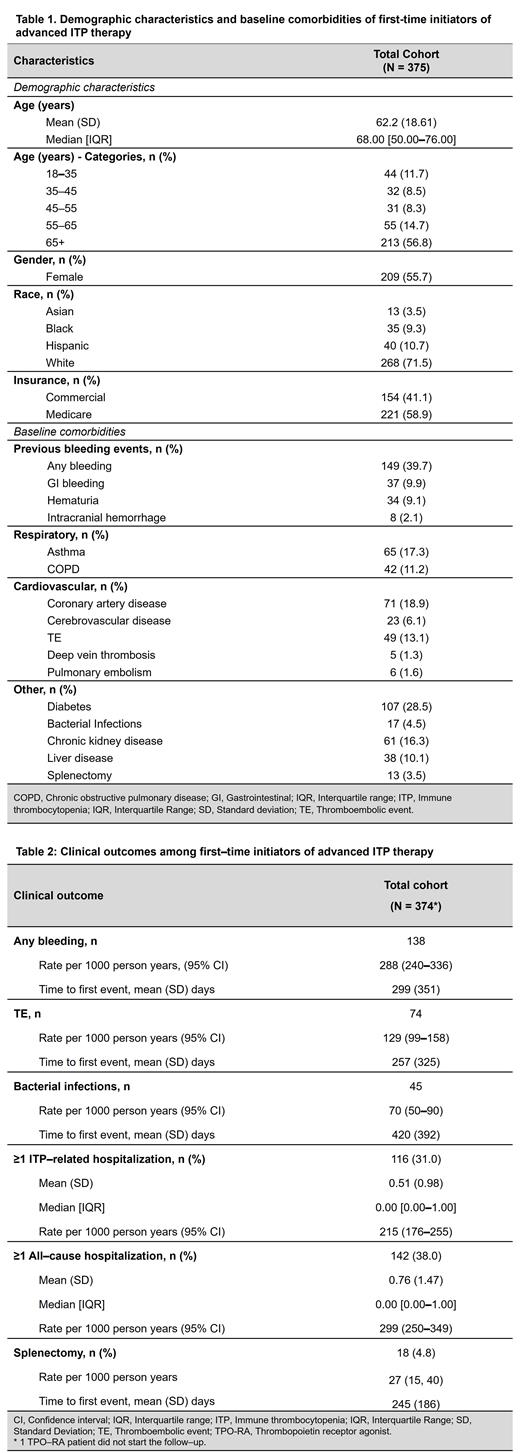
Total 375 patients with primary ITP lasting ≥3 months, who were first-time users of advanced therapies were included, with 51% (n=192) initiating TPO-RAs, 49% (n=182) initiating rituximab, and <1% (n=2) initiating fostamatinib. Mean (SD) age of overall population was 62 (19) years, and 55.7% were female. About 9% of patients were Black, 4% Asian, and 11% Hispanic ( Table 1).
In year prior to index date, previous bleeding events were most common clinical symptoms in patients with ITP initiating advanced therapies (any bleeding, 39.7%). Other frequently reported comorbidities were diabetes (28.5%), coronary artery disease (18.9%), and asthma (17.3%); 13.1% of patients had a TE. About 5% patients reported bacterial infections ( Table 1).
Patients initiating TPO-RA and those starting rituximab were observed for a median (interquartile range, IQR) duration of 22.2 (14.5-34.9) months and 27.1 (13.5-43.2) months, respectively. Almost 9% of TPO-RAs initiators switched to rituximab (median [IQR] time to switch: 3.2 [1.7-6.9] months), while 23.6% of rituximab initiators switched to TPO-RAs (median [IQR] time to switch: 3.1 [1.7-7.6] months) during follow-up.
After initiating advanced therapies, the rates of any bleeding event and TEs were 288 per 1000 person-years (95% CI: 240-336) and 129 per 1000 person-years (95% CI: 99-158), respectively. Mean (SD) time to first bleeding event was 299 (351) days and that of TE was 257 (325) days. Infection rate was 70 per 1000 person-years ( Table 2).
In follow-up, 31% of patients had ≥1 ITP-related hospitalization and 38% had an all-cause hospitalization, resulting in rates of 215 [95% CI: 176-255] and 299 [95% CI: 250-349] per 1000 person-years, respectively. Approximately 5% of patients underwent splenectomy (incidence rate: 27 per 1000 person-years [95% CI: 15-40]) with mean (SD) time of 245 (186) days post initiation of advanced ITP therapy ( Table 2).
Conclusion
This study demonstrated high clinical burden and need for subsequent additional therapies among patients with primary ITP lasting ≥3 months, which is evident from high rates of switching between advanced therapies, bleeding events requiring medical attention, TEs, and ITP-related hospitalizations.
Disclosures
Kuter:AIRx, Alexion (Syntimmune), Alnylam, Alpine, Amgen, argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, Cellularity, Cellphire, Chugai, CRICO, Daiichi Sankyo, Dianthus, Electra Therapeutics, Fuji, Hemopure, Hengrui. Immunovant, Incyte, Inmagenebio: Consultancy; Rubius: Current equity holder in publicly-traded company; AIRx, Alexion (Syntimmune), Alnylam, Alpine, Amgen, Argenx, BioCryst, Bristol Myers Squibb (BMS), Caremark, Cellularity, Cellphire, Chugai, CRICO, Daiichi Sankyo, Dianthus, Electra Therapeutics, Fuji, Hemopure, Hengrui, Immunovant, Incyte, Inmagenebio, Ke: Honoraria; UpToDate: Patents & Royalties: UpToDate Chapters; Platelet Disorder Support Association: Membership on an entity's Board of Directors or advisory committees; Kezar, Kyowa-Kirin, Merck Sharp & Dohme: Honoraria; Kezar, Kyowa-Kirin, Merck Sharp Dohme, Momenta, Novartis, Nuvig, Pfizer, Platelet Biogenesis, Platelet Disorder Support Association, Protagonist, Rigel, Sanofi (Bioveratif), Sanofi (Principia), Sanofi (Genzyme), Sobi (Dova), Takeda, UCB, Up-To-Date, Zafge: Consultancy; Alnylam, BioCryst, Novartis, Rigel, Sanofi (Principia), Takeda (Bioverativ), and UCB: Research Funding. Gouia:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Cordoba:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Ward:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Hemim:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Madziva:Sanofi: Current Employment. Petruski-Ivleva:Sanofi: Current Employment, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal